Risk Control - Safety Assessment
Risk Control - Safety Assessment
Overview
Safety evaluation of extractables and leachables (E&Ls) is important for the protection of patients inadvertently exposed to compounds from polymeric materials that interact with the drug substance and drug product. The focus of this white paper is to summarize the current regulatory landscape and other publications regarding the safety evaluation of drug substance and drug product impurities and highlight the opportunities that could be addressed with E&L specific guidance documents. Leachables are regarded as a subset of drug impurities and there are existing documents that can be leveraged for risk assessment purposes, summarized in Table 1.
.jpg)
The key aspects of existing impurity safety assessment guidance and specific considerations for leachables will be highlighted below.
What is needed? - framework for safety assessment of E&Ls
In general, the safety assessment approaches for E&Ls are consistent with that described for drug product and substance manufacturing process-related impurities. Therefore, building on existing guidelines, publications by Broschard et al. (2016) and Parris et al. 2020 (under revision) propose a safety evaluation process flow which relies on understanding the overall toxicological profile, including mutagenic potential to inform an appropriate control strategy. Leachables identified above an appropriate analytical threshold can be assessed for mutagenicity and other toxicity endpoints as shown in Figure 1.
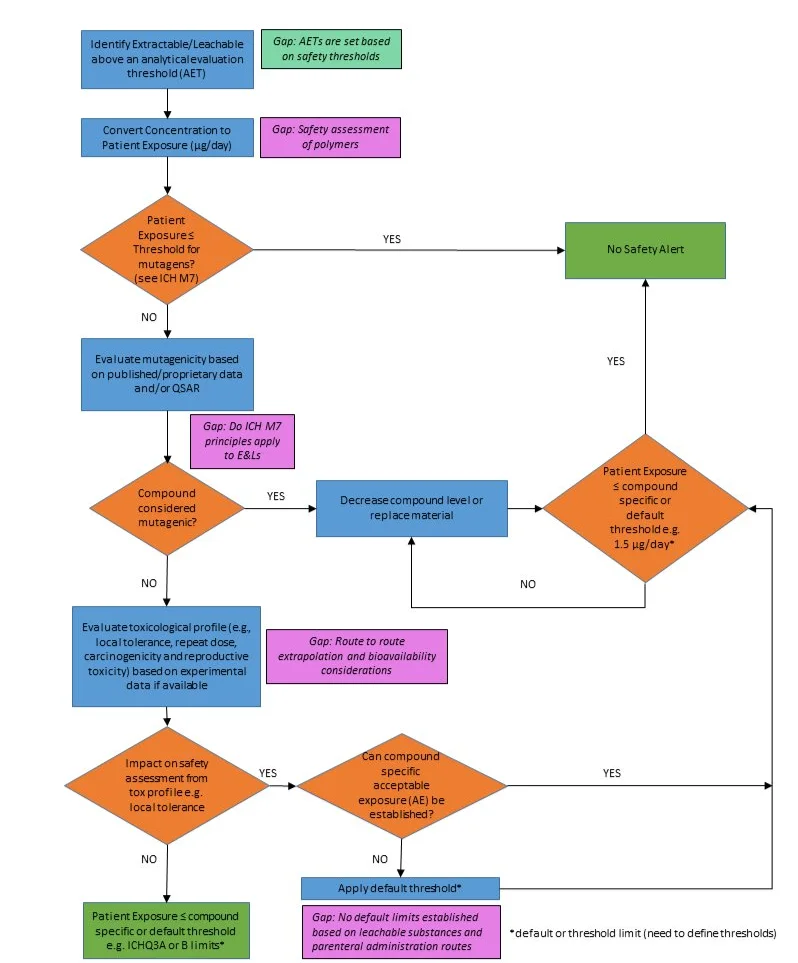
Figure 1. Example extractable/leachable safety evaluation process for a non-biologic parenteral, oral, or topical drug product. The flowchart addresses organic extractables/leachables. Note that this flowchart is an example of a suggested approach and highlights some of the specific considerations for E&Ls. It is illustrative of typical approaches, and it is recognized that other approaches can be used. Adapted from Broschard et al. 2016.
Special considerations for E&Ls
Aspects where additional guidance would be helpful in the safety assessment of E&Ls include but are not limited to:
Use of default thresholds for substances with limited toxicological data (further discussed in next section)
Application of M7 principles to extractables and leachables
Route of administration and bioavailability considerations
Evaluating endpoint-specific effects (e.g., irritation, sensitization)
Intermittent (non-daily) dosing and less than lifetime (LTL) limits for non-mutagenic substances
Risk assessment of polymers (e.g., monomers and other components)
A general framework for safety assessment of E&Ls includes a review of the available literature, and/or computational toxicology assessment to derive a substance-specific acceptable exposures (AEs). This is well described in Broschard et al. (2016) and Parris et al., 2020 (under review) and worked examples of acceptable exposures for common E&L substances are presented. In the absence of sufficient data, TTC concepts can been used to define acceptable exposures posing negligible risk to patients. Aspects of the framework warrant further consideration and guidance, for example extractable studies are performed based on screening of compounds whereas leachable studies are performed based on targets compounds. The extractable profile is likely to contain peaks that cannot be identified and/or quantified which is challenging for the toxicologist to perform the same level of safety assessment with extractable versus leachable profiles. In addition, computational structure activity relationship (SAR) evaluation of E&Ls may have limited value they can be out of domain of current knowledge, particularly for statistical systems.
Existing Default Thresholds
Default thresholds (i.e., doses where toxicity is not concerning for an untested chemical), are critical to E&Ls which oftentimes are not tested in a toxicity study. Toxicity testing of low-level E&Ls would result in significant animal usage with little value to patient safety. The TTC concept was originally developed for food safety as a pragmatic way to address potentially carcinogenic impurities in food contact materials as the Threshold of Regulation (TOR) by the U.S. Food and Drug Administration (US Food and Drug Administration, 1995). Subsequently, the use of this TTC has been expanded to encompass DNA reactive (mutagenic) impurities in pharmaceuticals and included in ICH M7 (ICH, 2014; ICH, 2017). TTC and less-than-lifetime (LTL) TTC values for several adverse health endpoints other than mutagenicity have been developed over the years and applied for food safety, pharmaceuticals, and personal healthcare products including those for systemic non-cancer effects (Dolan et al., 2005; Kroes et al., 2004), developmental and reproductive toxicity (Stanard et al., 2015), and dermal and respiratory sensitization (Safford et al., 2015; Safford et al., 2011, Carthew et al., 2009). The TTC can be used to judge whether exposure to a substance is low enough that the probability of adverse health effects is negligible, and no further data collection is necessary. TTCs are not applicable when adequate substance-specific assessment and toxicity data are available or are required under existing regulations (EFSA and WHO, 2016; EFSA Scientific Committee, 2012).
Challenges with the use of TTC and staged TTC approaches for E&L safety assessment include (Olson et al., 2016):
Whether a chemical being evaluated falls within the applicability domain of the toxicological database used to calculate the particular threshold value
Route-to-route extrapolation from one route of exposure (e.g., oral toxicity data) being applied to another (e.g., leachable substance in parenteral product)
The selection of a TTC is a crucial step in the safety assessment process and should consider available chemical data, such as structure and chemical class as well the dosing regimen of the drug product in question. For potentially DNA reactive (mutagenic) impurities without carcinogenicity data, ICH M7 establishes an acceptable intake, based on the TTC, of 1.5 μg/day for a lifetime (or LTL AIs for shorter duration exposures). Exposure below this AI is unlikely to exceed a lifetime cancer risk of 1 in 10exp5 for a mutagenic substance with unknown carcinogenic potential (ICH, 2017). ICH M7 recognizes a group of high potency mutagenic carcinogens referred to as the “cohort of concern” comprising aflatoxin-like-, N-nitroso-, and alkyl-azoxy compounds, for which acceptable intakes should be justified on a case by case basis..
For non-genotoxic chemicals, a tiered TTC approach may be applied to leachable substances based on potency/toxicity and potential for carcinogenicity. Examples of tiered and non-tiered TTC approaches include Harvey et al. (2017) and Dolan et al. (2005) (pharmaceuticals); Munro et al. (1996) and Kroes et al. (2004) (industrial chemicals and indirect food additives); Ball et al. (2007) and Paskiet et al. (2013) (E&L). The decision for selecting a TTC default value must consider the basis for how the TTC was derived and the relevance to the specific E&L substance being evaluated. For example, the TTC approach described by Dolan et al. (2005) was intended for APIs, but contained API data and also other types of chemicals such as those in the Munro et al. (1996) database or environmental contaminants. Munro et al. (1996) was intended for substances in food, and the relevance to parenteral exposure needs further development. Also, the relationship of these databases to the chemical space of E&Ls need to be further explored.
PQRI and US FDA
The Product Quality Research Institute (PQRI) proposed development of Safety Concern Thresholds (SCTs), and Qualification Thresholds (QT), for extractables and leachables for parenteral drug products (Paskiet et al., 2013). SCTs provide a threshold in which to identify E&Ls. The QT is a safety-based limit of 150 µg/day, where systemic toxicity (i.e., non-cancer) endpoints are not needed for the E&L. It should be acknowledged that substances exceeding the SCT are not necessarily “unsafe.” Such situations would need to be dealt with on a case by case basis based on scientific rational, the level of concern and the clinical indication. It should be noted that the SCT is based on mutagenic carcinogenicity, which for ICH M7 the AI is 1.5 µg/day. There are unofficial limits for E&Ls being derived and presented, such as the 50 µg/day limit for systemic toxicity of E&Ls in parenteral products (Paskiet, 2018 workshop presentation). PQRI also propose a threshold value of 5 µg/day for substances with known or suspected sensitization or irritation potential based on in silico alerts for dermal or respiratory sensitization. Whilst these limits have been presented orally at a workshop, they have not been formally published and the underlying data behind these limits are unclear. Therefore, the process for selecting the TTC based on route of administration of the pharmaceutical needs more clarity before adoption.
More recently, pharmacology/toxicology representatives from U.S. FDA Centre for Drug Evaluation and Research (CDER) have communicated recommendations or expectations regarding the identification and safety assessment of leachables in marketing applications for parenteral drug products. These recommendations are generally consistent with the PQRI proposal for small-volume parenteral products described above. One important difference is that FDA has not endorsed the 150 or 50 µg/day QT values for systemic toxicity proposed by PQRI (Paskiet, 2018 workshop presentation; Paskiet et al., 2013) and recommends a 5 µg/day threshold for general toxicity concern instead (McGovern, 2018; Mellon, 2019). This is consistent with the 5 µg/day PQRI concern threshold for sensitization and irritation endpoints, which for implementation purposes results in a single, 5 µg/day QT for all non-cancer concerns. Another notable point made in CDER presentations is that the 1.5 µg/day SCT for parenteral products can, in most cases, also be applied to inhalation products (McGovern, 2018; Robison, 2018). This recommendation is consistent with the principles described in ICH M7 and amends the more conservative 0.15 µg/day SCT described by PQRI for orally inhaled and nasal drug products (OINDPs) in 2007 (Ball et al., 2007).
It should be noted that the CDER recommendations have not been communicated in guidance documents. Therefore, it is unclear if these expectations are enforceable or will be consistently applied among various reviewers and divisions in the FDA. Finally, as shown in Table 2, there have been multiple limits that have been proposed, with different terminology making it difficult to navigate when setting analytical limits or understanding the safety of an untested chemical.
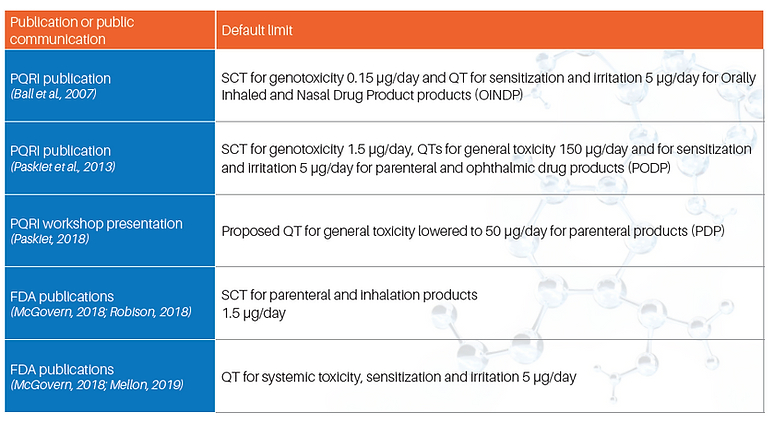
Figure 2: Conceptual representation of an E&L Hazard Appraisal framework.
Establishing Default Limits for Leachables
Default limits (i.e., TTC) have been readily adopted for impurities representing a wide range of chemicals; however, limits specific to leachable substances have not been established. A default or threshold value could be conservatively derived from a database of NOAELs (no-observed-adverse-effect-level) obtained in animal studies conducted for leachable substances representing a wide selection of toxicological endpoints and toxicokinetic effects. This approach would allow the establishment of threshold values based on applicable and relevant data for the assessment of E&L compounds without structural safety concerns and limited or no data, for which well-accepted threshold values already exist (e.g., TTC). Such limits would avoid overestimation of hazard for E&Ls with moderate to low toxicological concern, as may be anticipated with the conservative threshold recently communicated by the FDA (5 µg/day QT for all non-cancer concerns). Class-specific limits could be developed for common structural classes of E&Ls which are of lower concern. Sensitization needs to be further explored for its application to the default limit as the current assays were developed for skin sensitization, with limited application to parenteral exposure. Irritation also should be further explored as irritation/corrosion potential is often influenced by the acidic or basic physicochemical properties, which is less relevant for pH-controlled drug products.
The ELSIE consortium database compiles a reference dataset of leachable substances populated with available animal and human toxicity. The database comprises the name, synonyms and CAS number of the leachable substance, study type, species, sex, route of exposure, dose levels, study duration, endpoints reported, NOAEL and/or LOAEL and references. For each leachable substance, the ELSIE database will be reviewed and NOAEL values for relevant toxic effects will be curated and analyzed. The route of administration, species, bioavailability and quality of study will be specifically considered. This project is currently underway within ELSIE with the aim of developing a scientific-based QT approach applicable to non-mutagenic leachables substances.
Conclusion
E&Ls are considered a subset of drug impurities and therefore safety assessments should be conducted in accordance with current guidance, where the application of compound-specific or safety-based default threshold values such as TTCs are established (e.g., ICH M7, ICH Q3C and ICH Q3D). There are however specific considerations that apply to E&Ls that would greatly benefit from detailed and harmonized guidance. To support this, ELSIE is leading several scientific efforts to develop best practice in E&L safety risk assessment.
References
Ball, D., et al., 2007. Development of safety qualification thresholds and their use in orally inhaled and nasal drug product evaluation. Toxicol Sci. 97, 226-36.
Ball, D. J., Derivation of the Parenteral Safety Concern Threshold (SCT). PQRI PODP Extractables & Leachables Workshop, Rockville, MD, 2018.
Bercu, J. P., et al., 2018. Potential impurities in drug substances: Compound-specific toxicology limits for 20 synthetic reagents and by-products, and a class-specific toxicology limit for alkyl bromides. Regul Toxicol Pharmacol. 94, 172-182.
Broschard, T. H., et al., 2016. Assessing safety of extractables from materials and leachables in pharmaceuticals and biologics – Current challenges and approaches. Regul Toxicol Pharmacol. 81, 201-211.
Carthew et al., 2009. Exposure based waiving: the application of the toxicological threshold of concern (TTC) to inhalation exposure for aerosol ingredients in consumer products. Food Chem Tox 47; 1287–1295.
Dolan, D. G., et al., 2005. Application of the threshold of toxicological concern concept to pharmaceutical manufacturing operations. Regul Toxicol Pharmacol. 43, 1-9.
EFSA, WHO, 2016. Review of the Threshold of Toxicological Concern (TTC) approach and development of new TTC decision tree. EFSA Supporting Publications. 13, 1006E.
EFSA Scientific Committee, 2012. Scientific Opinion on Exploring options for providing advice about possible human health risks based on the concept of Threshold of Toxicological Concern (TTC). EFSA journal. 10, 2750.
European Centre for Ecotoxicology and Toxicology of Chemicals, The ECETOC Conceptual Framework for Polymer Risk Assessment (CF4Polymers). Brussels, 2019.
European Medicines Agency (EMA). Guideline on setting health based exposure limits for use in risk identification in the manufacture of different medicinal products in shared facilities EMA/CHMP/ CVMP/ SWP/169430/2012, 20 November 2014.
Harvey, J., et al., 2017. Management of organic impurities in small molecule medicinal products: Deriving safe limits for use in early development. Regul Toxicol Pharmacol. 84, 116-123.
Health Canada: Questions and Answers Request to evaluate the risk of the presence N-nitrosamine impurities in human pharmaceutical products, (October 2, 2019 letter from Health Canada to Market Authorization Holders).
ICH: Impurities In New Drug Substances Q3A (R2) (Current Step 4 version dated 25 October 2006).
ICH: Impurities In New Drug Products Q3B (R2) (Current Step 4 version dated 2 June 2006).
ICH Q3D Guideline for Elemental Impurities (Step 5; 25 July 2016). 2016a.
ICH, Impurities: Guideline for Residual Solvents Q3C (R6) (Current Step 4 version dated 20 October 2016). 2016b.
ICH, Assessment and Control of DNA Reactive (Mutagenic) Impurities In Pharmaceuticals To Limit Potential Carcinogenic Risk M7(R1). International Conference on harmonization of technical requirements for registration of pharmaceuticals for human use (ICH), Geneva, 2017.
Kroes, R., et al., 2004. Structure-based thresholds of toxicological concern (TTC): guidance for application to substances present at low levels in the diet. Food Chem Toxicol. 42, 65-83.
Li, K., et al., 2015. Creating a Holistic Extractables and Leachables (E&L) Program for Biotechnology Products. PDA J Pharm Sci Technol. 69, 590-619.
McGovern, T., Derivation and Validation of Parenteral Classification Strategy. PQRI PODP Extractables & Leachables Workshop, Rockville, MD, 2018.
Mellon, D., Nonclinical Review of Extractable Leachable Studies: Practical Advice from an FDA Reviewer. Extractables & Leachables USA, Arlington, VA, 2019.
Munro, I. C., et al., 1996. Correlation of structural class with no-observed-effect levels: a proposal for establishing a threshold of concern. Food Chem Toxicol. 34, 829-67.
Olson, M. J., et al., 2016. Issues and approaches for ensuring effective communication on acceptable daily exposure (ADE) values applied to pharmaceutical cleaning. Regul Toxicol Pharmacol. 79 Suppl 1, S19-27.
Parris et al., 2020. Considerations when deriving compound-specific limits for Extractables and Leachables from Pharmaceutical products: Four Case Studies. Regul Toxicol Pharmacol. under revision.
Paskiet, D., Introduction to PODP Recommendations: Risk Based Concepts. PQRI PODP Extractables & Leachables Workshop, Rockville, MD, 2018.
Paskiet, D., et al., 2013. The Product Quality Research Institute (PQRI) Leachables and Extractables Working Group Initiatives for Parenteral and Ophthalmic Drug Product (PODP). PDA J Pharm Sci Technol. 67, 430-47.
Patlewicz, G., et al., 2008. An evaluation of the implementation of the Cramer classification scheme in the Toxtree software. SAR QSAR Environ Res. 19, 495-524.
Robison, T. W., Application of Thresholds and Expectations: Regulatory Perspectives. PQRI PODP Extractables & Leachables Workshop, Rockville, MD, 2018.
Safford, R. J., et al., 2015. Extension of the Dermal Sensitisation Threshold (DST) approach to incorporate chemicals classified as reactive. Regul Toxicol Pharmacol. 72, 694-701.
Safford, R. J., et al., 2011. Refinement of the Dermal Sensitisation Threshold (DST) approach using a larger dataset and incorporating mechanistic chemistry domains. Regul Toxicol Pharmacol. 60, 218-24.
Stanard, B., et al., 2015. Threshold of toxicological concern (TTC) for developmental and reproductive toxicity of anticancer compounds. Regul Toxicol Pharmacol. 72, 602-9.
SwissMedic, Potential nitrosamine contamination: request to perform a risk evaluation. COVID-19: Deadline extension. Updated on 03.04.2020. https://www.swissmedic.ch/swissmedic/en/home/news/mitteilungen/aufforderung-zlinh, aberinnen-ham.html
US Food and Drug Administration, 1995. Food Additives; Threshold of Regulation for Substances Used in Food-Contact Articles. Final Rule. . 21 CFR Part 5.


